Dear all
Here is the latest in clinically relevant tendinopathy research
If your interested in tendinopathy clinical gems and tips check out upcoming courses in Melbourne, Canberra, Sydney and Perth.
All the best
Peter
Beeson performed a literature review of risk factors in plantar fasciopathy. Factors include BMI, obesity, limited DF, prolonged standing, hamstring and calf tightness. Beeson also suggests foot pronation on the foot posture index is associated with plantar fascia, however, Ribeiro et al. 2011 shows increased medial longitidunal arch in plantar fasciopathy.
www.sciencedirect.com/science/article/pii/S126877311400040X
Ribeiro, A. P., et al. (2011). “Rearfoot alignment and medial longitudinal arch configurations of runners with symptoms and histories of plantar fasciitis.” Clinics 66(6): 1027-1033
Masood et al. show increased EMG in soleus and FHL (no diff in gastroc) in Achilles tendinopathy during isometric contraction despite maintaining a lower force output. There was also higher glucose uptake (metabolic activity) in the Achilles tendon of painful participants. This suggests lower muscle efficiency and increased metabolism in Achilles tendinopathy.
www.ncbi.nlm.nih.gov/pubmed/24713192
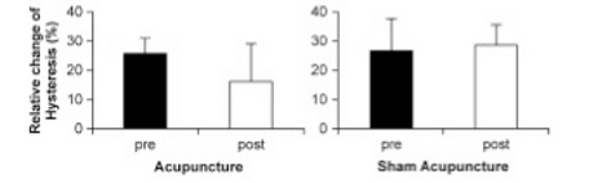
Interesting study by Fukushima et al. showing improved tendon properties (less hysteresis (see figure below), greater stiffness) following acupuncture of the gastrocnemius. No change in EMG. Had a sham acupuncture group so findings unlikely to be related to change in tendon properties from the testing procedures alone. How do these effects differ for soft tissue work?
www.sciencedirect.com/science/article/pii/S2211766014000139
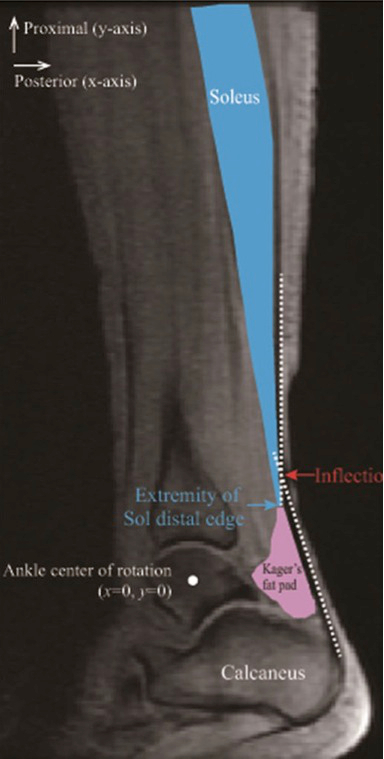
Kinugasa et al. look at Achilles bowing during plantar flexion using MRI and found it is near the junction of Kager’s fat and the distal soleus (see figure below), and depends on how low the soleus is. Fits in with clinical compression of Achilles, soleus and fat pad in plantar flexion, as described in recent Achilles diagnosis blog.
www.jfootankleres.com/content/7/S1/A43
Liem et al. show increase risk of rotator cuff tears (67% prevalence) among 55 patients with previous symptomatic contralateral cuff tears, compared with controls (11% prevalence). Underlying genetic predisposition or overuse due to dysfunction on contralateral side?
www.ncbi.nlm.nih.gov/pubmed/24500916
Dean et al. found increased expression of glutamate NMDAR1 receptor after one subacromial bursa steroid injection but not rotator cuff surgery. Glutamate induced pain and tissue pathology may partly explain poor long term effects of steroid injection.
bjsm.bmj.com/content/early/2014/03/27/bjsports-2013-093178.abstract?
Janssen et al. found that the main difference that could explain higher patellar tendon injury risk among elite vs sub-elite volleyball players was training volume, not landing kinematics, neuromuscular activation strategy, quads strength, patellar tendon load. Training hours per week and number of training sessions per week were associated – so volume of energy storage load is an issue, but why? Fluid flow?
http://onlinelibrary.wiley.com/doi/10.1111/sms.12206/abstract
Holm et al review surgical and non-surgical management and complication of Achilles tendon rupture – worth a read. 7 RCT’s were included. There was no difference in rerupture rates between treatments (range = 2-12%). There were generally better functional outcomes after surgery. Also report no difference in the risk of tendon elongation following surgery and conservative, but my clinical impression is more likely after conservative. For this reason I always recommend surgery, although surgeons seem less willing when there are muscle-tendon junction ruptures versus tendon midportion ruptures.
www.ncbi.nlm.nih.gov/pubmed/24650079
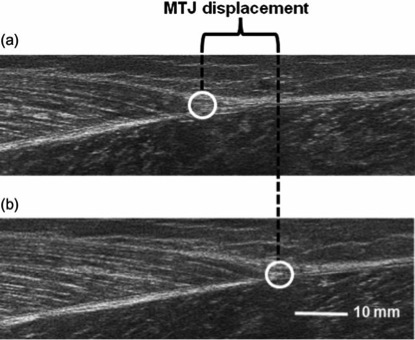
Konrad et al. – 49 participants performed PNF stretching for 6 weeks. Consisted of 15sec stretch followed by 6sec isometric contraction in standing calf stretch position against a wall. This was followed by 15sec of antagonist contraction. Entire procedure was repeated 4 times. Resulted in increased ankle DF range of motion and decreased tendon stiffness (i.e. greater stretch of the tendon measured at the muscle tendon junction with US on maximal isometric contraction – see photo below).
Mahieu et al. 2007 found similar decreased tendon stiffness effect with ballistic but not static stretching, although sustained static stretching (10 minutes) has the potential to reduce tendon stiffness (Kubo et al. 2001). Static and ballistic or PNF stretching may also have different effects on muscle force output. Ripe for a systematic review on tendon vs muscle effects of different types of stretching – any takers?
http://onlinelibrary.wiley.com/doi/10.1111/sms.12228/abstract
Mahieu N, McNair P, De Muynck M, Blanckaert I, Smits N, Witvrouw E. Effect of static and ballistic stretching on the muscle-tendon tissue properties. Med Sci Sports Exerc 2007: 39 (3): 494–501.
Kubo K, Kanehisa H, Kawakami Y, Fukunaga T: Influence of static stretching on viscoelastic properties of human tendon structures in vivo. J Appl Physiol. 2001, 90:520.
Very interesting study by Thorpe et al. – they harvested Achilles and horse digital flexor tendons (also an energy storage tendon) and apply preconditioning or fatigue (to point of some fascicle damage) loading cycles. The preconditioned cycles you could argue is like a ‘warm up’, and they displayed less compressive force, probably due to fluid flow out of the tendon, less viscosity, ie a more efficient tendon. Damaged tendons displayed less fascicle rotation (previously shown to be a unique quality of energy storage tendon fascicles – Thorpe 2013), and greater energy absorption in the tendon. This microstructural damage may be relevant to the development of tendon pathology. In vivo, the big question is what is it about energy storage load that influences mechanotransduction negatively and leads to injury – is it microstructural damage (as suggested in this study) or just a change in cell signaling prior to any structural damage? In vivo studies needed.
www.ncbi.nlm.nih.gov/pubmed/24747261
Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HR: Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: a possible mechanism for age-related tendinopathy. Eur. Cell. Mater. 2013, 25:48-60.
Rousseau et al. report good outcomes (72% returned to previous level running) for surgery for insertional Achilles pain among high level runners, but study highlights recovery is mean 9 months.
www.ncbi.nlm.nih.gov/pubmed/24748271
Murawski et al. retrospectively analysis outcomes of 32 patients treated with a single PRP injection after failing conservative treatment for Achilles tendinopathy. 78% were pain free at 6 months. Obvious limitations of retrospective, single group research, which I won’t harp on about, aside from saying it adds to the pile of these studies in PRP which really don’t tell us much! Participants were put in a boot for 2 weeks post injection, which is interesting. I would like to know more about the ‘failed’ conservative treatment that had prior to injection, as being stuck in a boot and then functionally rehabed for 8 weeks would have improved most even if the initial treatment was poor
http://fas.sagepub.com/content/early/2014/04/24/1938640014532129.full
Magnan et al. have published a systematic review of Achilles tendinopathy pathogenesis – an ambitious task. They review extrinsic and intrinsic risk factors, and key pathology events, including hypoxia, vascular endothelial growth factors and neurogenic inflammation. There is a proposal of hypoxia-centric pathogenesis, but there is evidence that cell signaling and other changes likely precede hypoxia
www.sciencedirect.com/science/article/pii/S126877311400037X
Lewis and Cook review the literature related to fluiroquinolone-related tendinopathy. Take home message – avoid use of these antiobiotics, especially in people with other known risk factors for tendinopathy.
www.ncbi.nlm.nih.gov/pubmed/24762232
Le et al. have investigate predictors of retear among an impressive 1000 cases of rotator cuff surgical repair. At 6 months there was a 17% retear rate and the best predictors were tear size & thickness, age at surgery. It would be interest to see whether acute (i.e. associated with definite incident) versus degenerative tears have a different prognosis and re-tear rate – this was not measured.
ajs.sagepub.com/content/early/2014/04/18/0363546514525336.abstract
Fascinating study by Rosengarten et al. showing structural tendon changes with UTC at 2 days post maximal loading (an AFL football game) that return to normal at day 4. Authors suggest this may reflect cell driven, possibly upregulation of ground substance, which fits with the Cook-Purdam Reactive-degenerative tendinopathy model.
bjsm.bmj.com/content/early/2014/04/15/bjsports-2013-092713.full